Graphene shows promise for next-generation rechargeable batteries
Graphene Flagship researchers show how the 2d material graphene can improve the energy capacity, efficiency and stability of lithium-oxygen batteries.
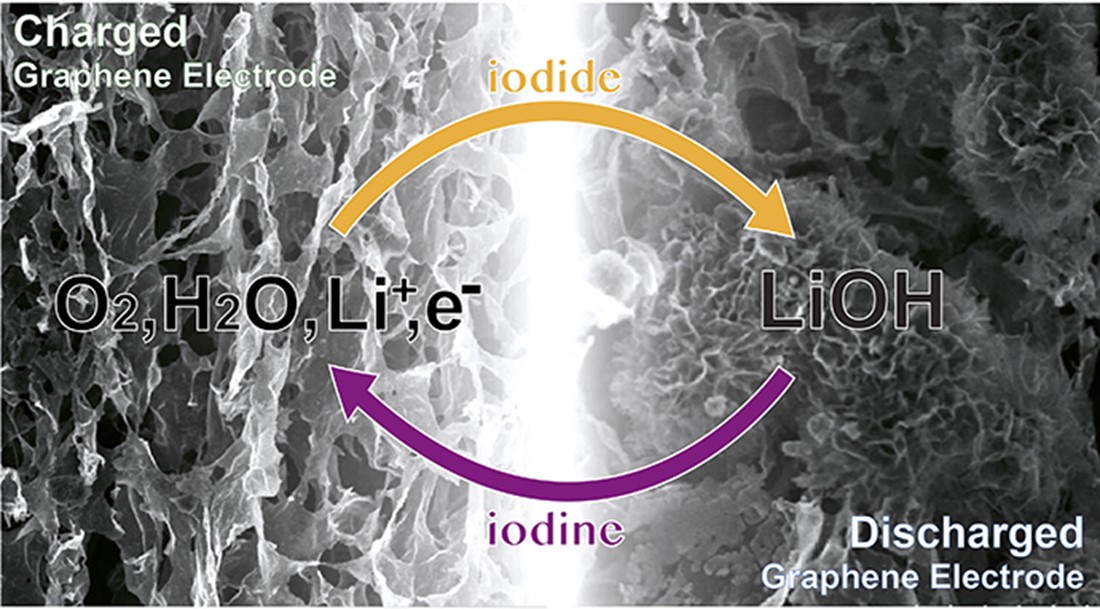
Graphene-based battery electrode superimposed with a schematic of iodide-mediated chemistry underpinning the charge-discharge cycle (image: copyright © 2015 Tao Liu, Clare Grey & Gabriella Bocchetti/University of Cambridge).
Scientists in Cambridge, UK, have demonstrated a lithium-oxygen battery with high energy density, efficiency and stability. The device is over 90% efficient, and may be recharged more than 2,000 times. The results of the research study led by Cambridge University chemist Clare Grey, who leads the energy activities in the Graphene Flagship, have been published in the journal Science.
The Graphene Flagship is a pan-European consortium of academic and industrial partners designed to take graphene and related 2d materials from the laboratory to factory floor and beyond.
The rechargeable batteries that power today's mobile devices are based on lithium-ion technology, and application demands are outstripping the relatively low energy capacities of lithium-ion cells. Lithium-oxygen, or lithium-air, cells, on the other hand, have a theoretical energy density some 10 times that achievable with lithium-ion chemistry.
To give you an idea of what this means in practice, lithium-oxygen batteries could in principle have an energy density comparable with that of gasoline. This would enable an electric car with a battery one-fifth the cost and one-fifth the weight of those currently on the market to travel the 650 kilometres between London and Edinburgh on a single charge. However, as is the case with other next-generation batteries, there are several practical challenges that must be addressed before lithium-air batteries can present a viable alternative to gasoline as an energy source for road vehicles and other high power applications.
Researchers from the University of Cambridge have demonstrated how a few of the obstacles may be overcome. In their lab, they have developed a lithium-oxygen demonstrator cell with a higher energy capacity, increased efficiency and improved material stability over that of previous attempts.
The Cambridge lithium-oxygen cell features a porous, ‘fluffy’ electrode made from graphene – the single-atom-thick crystalline allotrope of carbon, with the carbon atoms arranged in a honeycomb-like lattice – together with additives that alter the chemical reactions at work in the battery. While the results of the latest study are promising, Grey and her colleagues caution that a practical lithium-air device remains at least a decade away.
“What we've achieved is a significant advance for this technology, and suggests whole new areas for research – we haven't solved all the problems inherent to this chemistry, but our results do show routes forward towards a practical device,” says Grey.
Many of the technologies we use in everyday life have with time been getting smaller, faster and cheaper – with the notable exception of batteries. Aside from the possibility of a smartphone that can last several days on a single charge, the challenges associated with making better rechargeable batteries are holding back the widespread adoption of two major clean, green technologies: electric cars, and grid-scale storage for solar power.
“In their simplest form, batteries are made of three components: a positive electrode, a negative electrode and an electrolyte,” says Tao Liu, first author of the Science paper.
In the lithium-ion batteries that power laptops and smartphones, the negative electrode (cathode) is made of graphite, and the positive electrode (anode) a metal oxide such as lithium cobalt oxide. The electrolyte is a lithium salt dissolved in an organic solvent, and it is movement of lithium ions between the electrodes that drives the battery current. Lithium-ion batteries are lightweight, but their capacity deteriorates with age, and the relatively low energy densities mean that they must be recharged frequently, as any frustrated mobile phone user will attest.
Over the past decade, researchers have studied various alternatives to lithium-ion cells. Owing to their relatively high energy density, lithium-air batteries are considered the ultimate in next-generation electrical energy storage, but previous attempts have suffered from relatively low efficiency, poor performance, and the presence of deleterious chemical reactions. Another problem is that they can only be cycled in a pure oxygen atmosphere. This, when the air we breathe is mostly nitrogen, with less than 21% oxygen by volume.
What Liu, Grey and their colleagues have developed relies on a very different chemistry than earlier attempts at a non-aqueous lithium-air battery. With the addition of water and the use of a lithium iodide mediator, the Cambridge battery shows fewer of the chemical reactions that degrade battery cells, making it far more stable following multiple charge-discharge cycles.
By precisely engineering the structure of the electrode, switching from graphite to a highly porous form of graphene, adding the lithium iodide mediator, and altering the chemical makeup of the electrolyte, the researchers were able to reduce the voltage gap between charge and discharge to just 0.2 volts. The smaller this voltage gap, the more efficient the battery. Previous attempts at a lithium-air battery have only managed to reduce the gap to 0.5–1.0 volts, whereas 0.2 volts is closer to that of a lithium-ion cell, and equates to an energy efficiency of 93%.
Other issues still to be addressed include finding a way to protect the metal electrode so that it does not lead to the growth of spindly lithium metal fibres known as dendrites, which can cause batteries to explode if they short-circuit the cell. Additionally, as with previous attempts at lithium-oxygen cells, the demonstrator cell can only be cycled in a pure oxygen environment. Atmospheric carbon dioxide, nitrogen and moisture are all damaging to the metal electrode.
“There's still a lot of work to do,” says Liu. “But what we've seen here suggests that there are ways to solve these problems – maybe we've just got to look at things a little differently.”
Grey considers the path ahead: “While there are still plenty of fundamental studies that remain to be done, to iron out some of the mechanistic details, the current results are extremely exciting – we are still very much at the development stage, but we've shown that there are solutions to some of the tough problems associated with this technology.”
The technology has been patented, and will be promoted through Cambridge Enterprise, the commercialisation arm of the University of Cambridge.
Reference
Liu et. al., Cycling Li-O2 batteries via LiOH formation and decomposition, Science (2015). doi:10.1126/science.aac7730



