Chrysalis-shaped graphene oxide cathodes make magnesium batteries cleaner and greener
Chrysalis-shaped graphene and vanadium microstructures boost the performance of magnesium-based batteries as a sustainable alternative to lithium
Scientists at Graphene Flagship partners the University of Padova, the University of Trieste and CNR-IMM, Italy, in collaboration with researchers from other European institutions, have developed a new strategy to boost the performance of magnesium-based rechargeable batteries. Combining vanadium and graphene oxide, they obtained a high-power cathode that shows excellent promise for sustainable energy storage.
Rechargeable batteries are widespread in modern electronics, as they can repeatedly accumulate, store and discharge energy through a reversible electrochemical reaction. This makes them vital for the lasting function of mobile phones, laptops and electric vehicles, all of which endure hundreds of charge cycles over their lifetime. Typical rechargeable batteries are made using lithium anodes, but magnesium anodes have a number of properties that make them promising alternatives.
"Several factors make magnesium-based rechargeable batteries attractive," begins first author Vito Di Noto, from Graphene Flagship partner the University of Padova, Italy. "They have a higher volumetric capacity than those made with lithium, and they can be safely handled in air." Moreover, magnesium is a cheaper and more abundant raw material. "In fact, it is one of the most abundant elements in the Earth's crust," explains Di Noto. Magnesium anodes also represent a safer alternative: they are less prone to dendrite formation, a phenomenon that can lead to short circuits and, in rare circumstances, battery explosion.
However, the development of magnesium batteries has been hindered by their poorly performing cathodes, which often result in significantly worse-performing devices than their lithium-based counterparts.
To tackle this challenge, the researchers developed an all-new cathode material for magnesium batteries based on graphene and vanadium oxides. The material exhibits a peculiar chrysalis-like microstructure that enhances the performance of the battery. Graphene oxide flakes encircle a nanoparticle core based on vanadium oxide: "the structures are fixed together thanks to a layer of ammonium ions," explains Di Noto. The chrysalis-like material combines vanadium's high redox activity and graphene oxide's electrical properties. "This yields a cathode with very strong chemical and electrochemical stability," he continues.
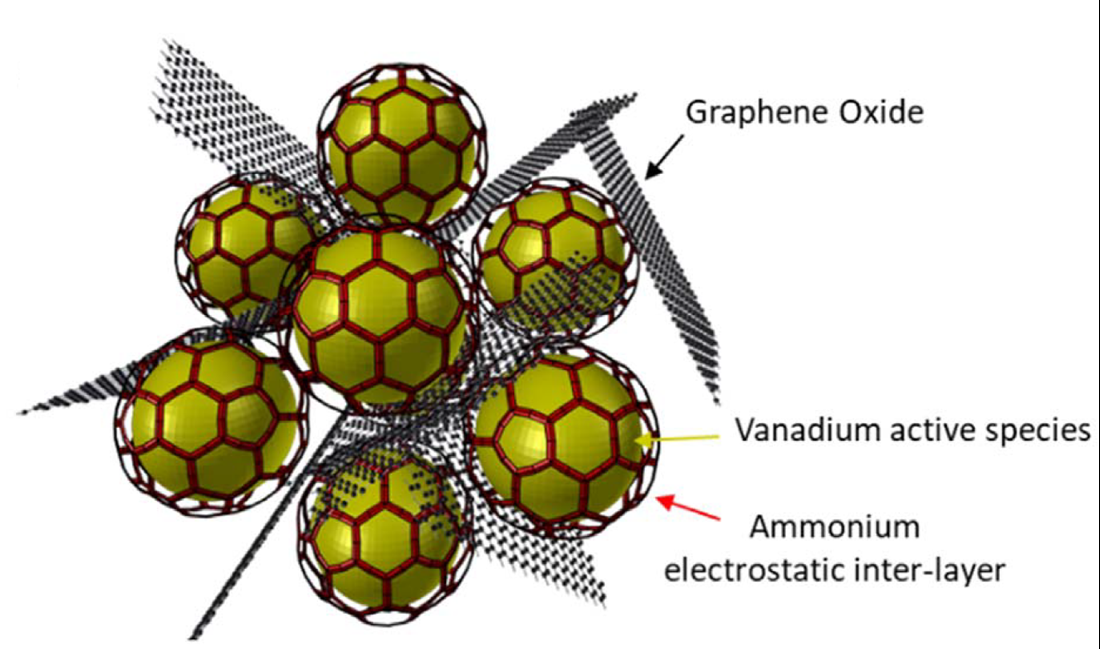
The new graphene-enhanced cathode has allowed researchers to operate a coin cell at very high current rates and power, with a promisingly high specific capacity. "The synergistic effects provided by graphene oxide, vanadium and the chrysalis morphology enable the coin cell to operate with 500% more sustained current than state-of-the-art magnesium batteries, at a 40% higher working potential." These properties could be exploited to make batteries for mobile devices that last longer between charges or deliver more power.
Furthermore, magnesium's high natural abundance means that magnesium-based rechargeable batteries could be an environmentally friendly solution. This work brings graphene batteries one step closer to the market. "Magnesium is one of the most sustainable metals in the world, and can be easily recycled – up to 100%," Di Noto continues. "We hope that our work will contribute to the turning point towards the establishment of a greener and more sustainable energy economy."
Daniel Carriazo, Graphene Flagship Work Package Deputy for Energy Storage, comments: "As the production of lithium-ion batteries increases exponentially to fulfil the demand of new applications, it is necessary to develop alternative energy storage technologies made out of accessible and environmentally friendly materials." Carriazo says that this work shows very promising results when a vanadium-based graphene composite is used as the positive electrode in a potassium-ion battery. "The incorporation of graphene enables fast charging, overcoming one of the limitations associated with this technology," he continues.
Andrea C. Ferrari, Science and Technology Officer of the Graphene Flagship and Chair of its Management Panel, adds: "Graphene and layered materials have recognised potential in energy storage, and graphene is already present in commercial devices. This approach tackles the need to produce more environmentally sustainable batteries, and thanks to the introduction of graphene oxide into the cathode, shows how magnesium could be used, which is easier to recycle. Sustainable development always guides the technology and innovation roadmap of the Graphene Flagship, and this research is yet another promising example."
References
- Gioele Pagot et al., J. Electrochem. Soc., 167, 070547 (2020)




