Graphene acid helps remove heavy metal pollutants from water
Graphene Flagship Partnering Project 2D-CHEM expands the potential of graphene derivatives for societal challenges like producing clean water.
Researchers at Graphene Flagship Associated Member Palacký University Olomouc in the Czech Republic, involved in our Partnering Project 2D-CHEM, have shown that graphene decorated with carboxylic groups can be used to extract or remove different types of metallic pollutant from water. Funded by a European Research Council (ERC) consolidator grant, this Partnering Project seeks to enhance our understanding of the chemistry of fluorographene and other layered materials in order to produce a broad range of graphene derivatives with a multitude of properties.
The World Health Organization estimates that half of the world’s population will be living in water-stressed areas by 2025. Contamination with heavy metals is among the primary threats to aquatic ecosystems and human health. The most economically advantageous method for metal removal from water is sorption on suitable porous organic and inorganic sorbents. However, current sorption technologies are often inefficient in capturing heavy metals and non-reusable. In this study, the team succeeded in producing a sorbent with graphene acid, an ultra-thin organic acid that offers excellent biocompatibility, conductivity and dispersibility in water.
This achievement comes after years of working on strategies to chemically synthetise graphene derivatives that cannot be obtained by direct functionalisation of graphene itself. The researchers prepared graphene acid starting from fluorographene, using a technique developed at the Graphene Flagship Associated Member Palacký University Olomouc: they replaced fluorine with other chemical elements to obtain the desired graphene derivative. For example, graphene acid was prepared via a two-step process: first fluorographene was transformed into fluorine-free cyanographene, and then it was hydrolysed to graphene acid.
“In principle, we can introduce any group to the surface of graphene: carboxyl, amino, sulfhydryl and hydroxyl groups, creating new materials that can be employed in a wide spectrum of applications, ranging from metal extraction, electrochemical sensing, magnetism, catalysis and energy storage,” says Michal Otyepka, 2D-CHEM leader and Head of the Regional Centre of Advanced Technology and Materials (RCPTM) - the Czech Advanced Technology and Research Institute (CATRIN) at Graphene Flagship Associated Member Palacký University Olomouc.
The researchers reported that graphene acid-based water sorbents bind and capture heavy metals with high affinity, and without leaching. Furthermore, the sorbent works also in the presence of other ions, which are naturally found in drinking water, and can also be reused after pH treatment without loss of performance.
“We obtained outstanding efficiencies in, removing highly toxic lead or cadmium from water, for example, reducing them down to levels allowed for drinking water,” explains Otyepka.
The researchers showed that this graphene acid-based sorbent not only can remove heavy metals from contaminated waters, but can also serve to extract noble metals, such as palladium, gallium or indium. The supply of noble metals on our planet is limited, and their inexpensive and efficient separation from waters provides enormous industrial opportunities.
“I am very proud that the Graphene Flagship Partnership Division contributes significantly to the accomplishment of the UN Sustainable Development Goals. By improving the effectiveness of graphene-based sorbents, the 2D-CHEM project has made an important step toward the availability and sustainable management of water and sanitation for all. The latter also offers an attractive opportunity for noble metal recovery and catalysts reusability with zero losses in performance,” adds Yuri Svirko from Graphene Flagship Associated Member University of Eastern Finland, Partnering Division leader.
References
Chronopoulos, Demetrios D., et al. "Chemistry, properties, and applications of fluorographene." Applied materials today 9 (2017): 60-70. https://www.sciencedirect.com/science/article/pii/S2352940717301129?via%3Dihub
Bakandritsos, Aristides, et al. "Cyanographene and graphene acid: emerging derivatives enabling high-yield and selective functionalization of graphene." ACS nano 11.3 (2017): 2982-2991. https://pubs.acs.org/doi/10.1021/acsnano.6b08449
Kolařík, Jan, et al. "Carboxylated Graphene for Radical-Assisted Ultra-Trace-Level Water Treatment and Noble Metal Recovery." ACS nano 15.2 (2021): 3349-3358. https://pubs.acs.org/doi/abs/10.1021/acsnano.0c10093
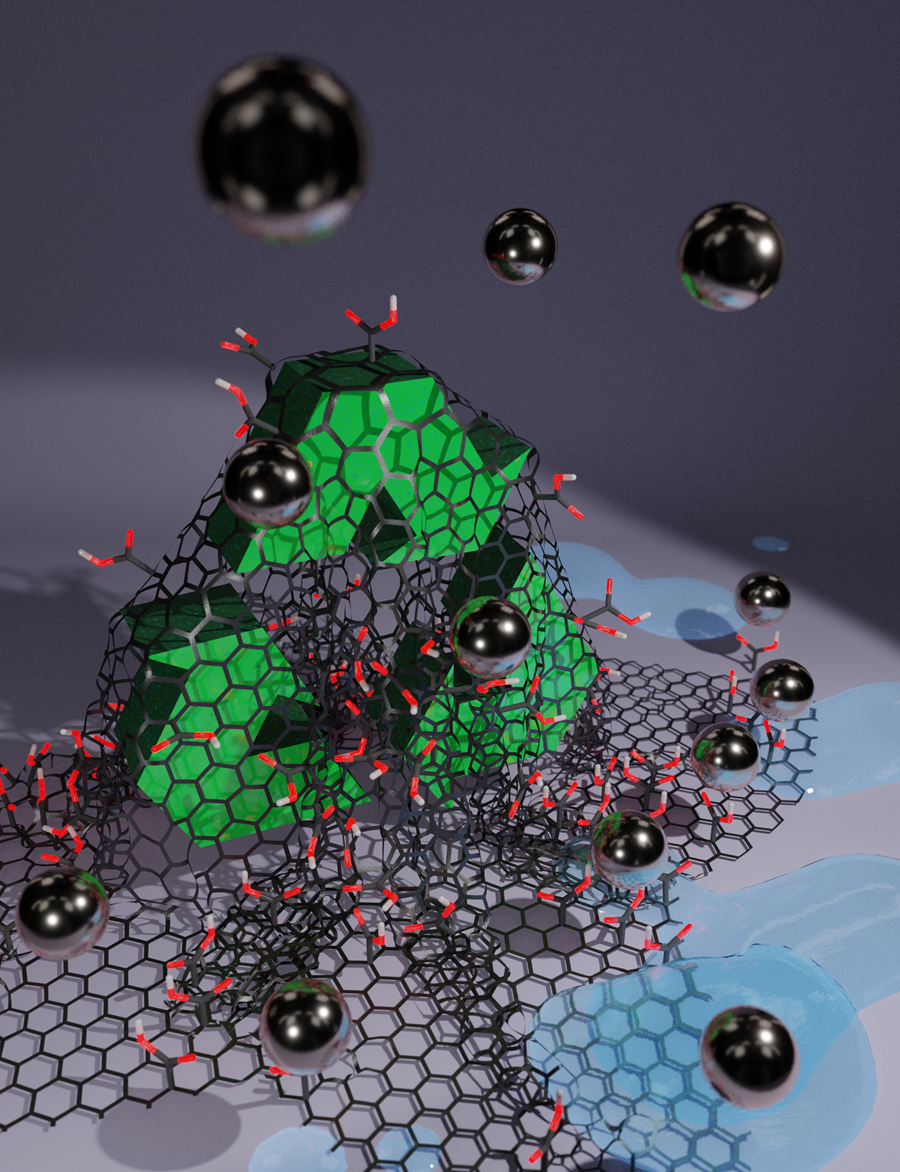
Reusable water sorbents based on graphene acid remove heavy metals from water. Credit: Martin Pykal




