Ultra-thin 'teflon' to reactivate graphene
The sunrise of new graphene derivatives is achieved by chemistry of fluorographene.
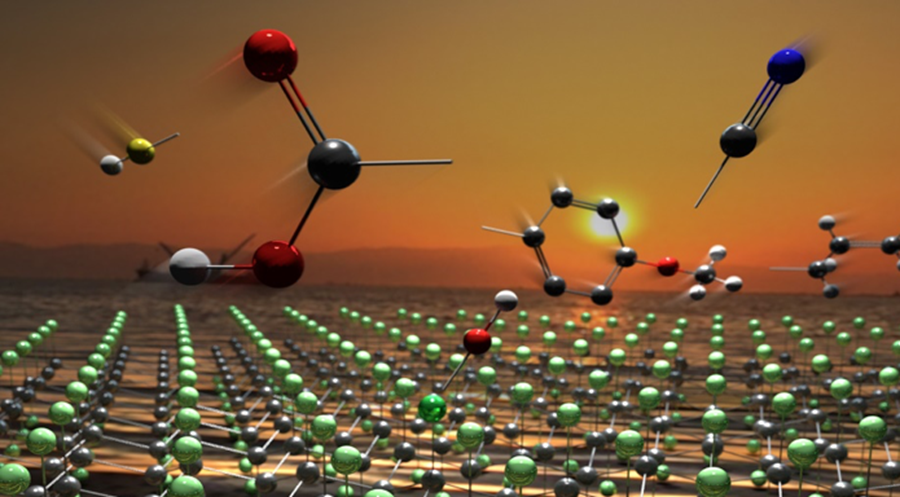
The sunrise of new graphene derivatives is achieved by chemistry of fluorographene. Credit: Martin Pykal
Fluorographene is a graphene derivative with fluorine atoms linked to the carbons. Fluorine atoms make fluorographene an electrical insulator. This compound can be imagined as an ultra-thin version of teflon – technically called polytetrafluoroethylene. Teflon is also formed by carbon and fluorine atoms. Hence, both are perfluorocarbons, but with different chemical formulas and structures.
'Despite the chemical similarities, there is a particular difference: fluorographene carries the fluorines bounded to tertiary carbons,' explains Michal Otyepka, a researcher at Graphene Flagship associated member Palacký University (Czech Republic). 'Tertiary carbons are attached to three other carbons, and they are considered the Achilles heel of perfluorocarbons,' adds Otyepka. Researchers took advantage of this chemical vulnerability, and used this material to create new functionalised graphene derivatives.
Normally, the chemical bond between carbon and fluorine is very strong, one of the most difficult to break. That is why perfluorocarbons are very stable and inert products – the very reason why we use Teflon to protect all sort of materials. However, the tertiary fluorine-carbon bond is susceptible to chemical reactions.
'In 2010, we demonstrated that fluorographene can be transformed into graphene, and we attributed this to the presence of this type of carbon-fluorine bond,' explains Otyepka. 'Since then, we have analysed several reaction channels or methods, which allow the elimination of fluorines, as well as their replacement with other chemical elements,' he adds.
Now, researchers within the Graphene Flagship uncovered that different types of solvent can favour different reaction paths. Carefully choosing the solvents for the reaction, chemists can control the chemical composition of the final material. 'This finding, published in the Journal of Physical Chemistry Letters, enables an elegant way for fine tuning the final properties of the graphene derivative,' explains Otyepka.
This research is part of the European 2DCHEM project (Two-Dimensional Chemistry towards New Graphene Derivatives), a Partnering Project of the Graphene Flagship, whose objective is to understand and control the chemistry of fluorographene and other 2D materials to produce graphene derivatives. These new materials can then be used in a wide spectrum of applications: electrochemical sensing, magnetism, separation technologies, catalysts, and energy storage.
The latest results and future challenges in this field will be presented by Otyepka on September 10th during the Graphene Week 2018, an annual congress co-organised by the Graphene Flagship initiative of the European Union, which this year is held from 10 to 14 September in San Sebastian (Spain).
References:
Dagmar Matochová, Miroslav Medved', Aristides Bakandritsos, Tomáš Steklý, Radek Zbořil and Michal Otyepka. "2D Chemistry: Chemical Control of Graphene Derivatization". J. Phys. Chem. Lett., 2018, 9 (13), pp 3580–3585. DOI: 10.1021/acs.jpclett.8b01596
SINC produces scientific news for the European project SCOPE, coordinated by FECYT and funded by the European Union through Horizon 2020, its funding program. The SCOPE mission is to communicate visionary research results of partnering projects in the framework of the Graphene Flagship and the Human Brain Project, as well as to enhance the FET Flagships partnering environment in the European Union.
This is an edited version of a piece produced by SINC under a Creative Commons Licence.



