Graphene oxide biodegrades with help of human enzymes
Graphene Flagship researchers show how graphene oxide suspended in water biodegrades in a reaction catalysed by a human enzyme, with the effectiveness of the breakdown dependent on the colloidal stability of the suspension. The study should guide the development of graphene-based biomedical applications.
As with all new materials with industrial promise, there is considerable expert and public interest in the health and safety aspects of graphene. The development and commercial exploitation of graphene is at an early stage, and its environmental, health and safety risks are being investigated by researchers linked with Europe’s Graphene Flagship. The flagship is a large international consortium of academic and industrial partners, part-funded by the European Commission, that focuses on the need for Europe to address the big scientific and technological challenges through long-term, multidisciplinary research efforts.
The potential health and safety impacts of graphene and other two-dimensional materials are a focus of intensive research efforts. Environmental persistence and accumulation are key issues when it comes to the exploitation of graphene-based materials, and safely disposing of these and other engineered materials at the end of their useful lives is of particular interest. When it comes to graphene specifically, the oxidised form of this two-dimensional allotrope of carbon holds considerable promise for use in drug delivery, bio-imaging, tissue engineering, biosensing and a number of other related applications, owing to its high aqueous dispersibility and biocompatbility.
If graphene oxide is to have a productive role in biomedical technologies, its toxicological effects must be systematically evaluated. Studies have shown that graphene oxide can in some situations damage living cells and attenuate immune response. When considered together, however, the results of the various experiments carried out to date are inconclusive, and in some cases contradictory.
Graphene and its various compounds may be biocompatible, but very little has been reported about biodegradation. To that end, flagship researchers led by Alberto Bianco, an organic chemist at the French National Research Council in Strasbourg, have looked in detail at the biodegradation of graphene oxide by an enzyme. Reporting their results in in the journal Small, the researchers show that myeloperoxidase – derived from human white blood cells in the presence of a low concentration of hydrogen peroxide – can completely metabolise graphene oxide in the case of highly dispersed samples.
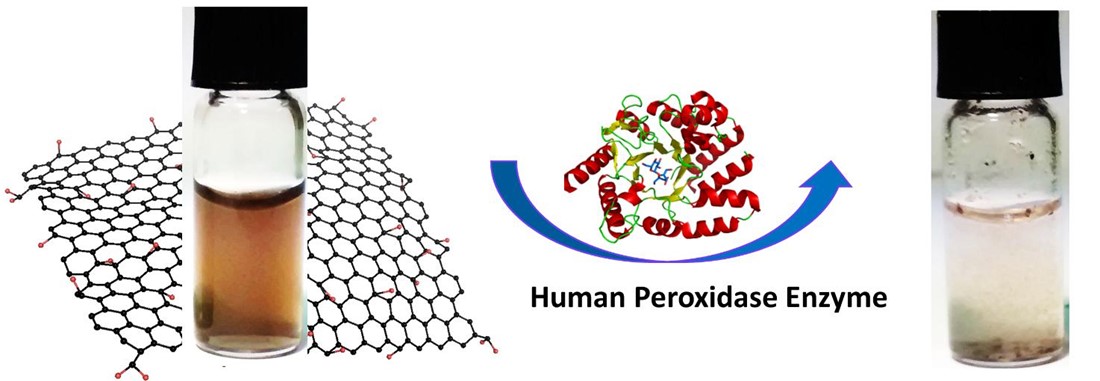
Graphene oxide suspensions before and after degradation by a human enzyme (image copyright © 2015 Rajendra Kurapati/CNRS).
The first author of the Small paper is Rajendra Kurapati, a postdoctoral researcher in Bianco’s group. Kurapati and his colleagues focused their attention on the capacity of myeloperoxidase to degrade three graphene oxide samples, classed according to degree of dispersibility in water. Important to note is that we are talking here about dispersibility rather than material concentration. What the researchers found is that highly aggregated suspensions of graphene oxide fail to biodegrade in the presence of myeloperoxidase, but the more stable colloids are completely broken down by the enzyme. In chemical terms, the dispersibility of graphene oxide depends on the oxygen groups present on the graphene surface, and this in turn relates to biodegradation.
After detailing the findings of their experiment, the researchers discuss the mechanism of graphene oxide degradation, beginning with a summary of the process by which myeloperoxidase acts against infection by microbes and other invasive materials that inflame biological tissues. During the inflammation process, neutrophils, a subtype of white blood cells, gather at the infection site and secrete myeloperoxidase, which catalyses a chemical reaction with chloride ions and hydrogen peroxide to produce strong oxidants such as hypochlorous acid. These oxidants have antimicrobial qualities, and are also known to degrade polyester-based implants, extracellular sugars and oxidised carbon nanotubes.
The study authors suggest that the high redox potentials of oxidants produced in the myeloperoxidase-catalysed reaction could in a similar way degrade graphene oxide held in suspension. Material breakdown likely starts at the level of carbon atoms connected with oxygen in the graphene lattice, and central to this is the hypochlorous acid produced in the reaction. Surface electric charge is also thought to contribute, as it does in the case of oxidised carbon nanotubes, since it favours the strong binding of graphene oxide with the enzyme, and subsequently triggers its degradation.
“Our study demonstrates the complete breakdown of graphene oxide by myeloperoxidase, and the results indicate that accidental inhalation of graphene oxide presents a manageable health risk in humans and other species,” says Bianco. “On the other hand, the translation of graphene-based materials into clinically safe biomaterials for biomedical applications is also judged by biodegradability. Our research may provide a method for the environmentally safe disposal of graphene-based materials. It could also guide the development of biocompatible graphene-based carriers for the delivery of bioactive molecules.”
The detailed mechanism for graphene oxide biodegradation is a subject for further investigation, but the findings of the latest study are clear. Graphene oxide breaks down in the presence of hydrogen peroxide, in a reaction catalysed by the myeloperoxidase enzyme. The degree of degradation depends on the colloidal stability of the suspension, which indicates that the hydrophilic nature of graphene oxide is a key factor in its breakdown by enzymes. Colloidal stability should therefore be considered when engineering graphene oxide materials for biomedical applications.
Further reading
Kurapati et al., Dispersibility-dependent biodegradation of graphene oxide by myeloperoxidase, Small, 8 May 2015; doi:10.1002/smll.201500038.



