SMENA Catalysis AB develops layered materials with bespoke edges for gas sensing and hydrogen catalysis
The Swedish spin off pioneers a technology to control edges in transition metal dichalcogenides with close-to-atomic precision
Timur Shegai’s and Batulga Munkhbat’s research on transition-metal dichalcogenides (TMDs) led to the creation of SMENA Catalysis AB in 2021. This Graphene Flagship Associated Member is a spin-off of Graphene Flagship Partner Chalmers University of Technology in Sweden. The team discovered a method to improve the properties of layered molybdenum disulfide (MoS2), by introducing highly controllable edges and holes in the material.
Shegai tells us about his company’s products and future directions.
How did it all start?
After showing promising results on a lab scale, SMENA Catalysis AB was formed with support from the Chalmers Innovation Office and Chalmers Ventures. After the first CEO was appointed, the company was quickly accepted into the award-winning Chalmers Venture Accelerator program and secured its first pre-seed funding as well as administrative and business development support. Currently, the company has expanded its workforce and has initiated its first collaboration with industry actors.
What do you focus on?
We have two business segments: Gas Sensors and Hydrogen Catalysis, that use our processed MoS2-based material, Molybdenyx.
For our Gas Sensors, we supply our customers with gas sensing elements, think of them as small microchips. The chemical reaction is read by a detector which deciphers the concentration of and the type of gas.
For our Hydrogen Catalysis segment, we provide Molybdenyx in a powder format, so our customers can apply it to Proton Exchange Membrane (PEM)-Electrolysers, in order to promote the chemical reaction that breaks water into hydrogen and oxygen and creates hydrogen gas (electrolysis).
What are your unique selling points?
In the case of gas sensing, metal oxides and carbon nanomaterial-based gas sensors are hampered by high temperature requirements, high power consumption, high sensitivity to humidity variations, low sensitivity, and incomplete recovery at room temperature. Metal nanoparticle sensors can be “poisoned” by gas molecules, decreasing their ability to detect target molecules. Finally, conducting polymers can work at room temperature, but they are highly sensitive to humidity. Our edge-enriched MoS2 material contains a large number of active sites, i.e. our material has plentiful hexagonal holes. These active sites absorb gas molecules with higher sensitivity, more specific interaction with the selected gas, and do not suffer from humidity changes. This enables the combination of benefits that could not be combined before, such as sensitivity, selectivity, accuracy and low energy consumption in a tiny sensor.
MoS2 is also an abundantly available and naturally occurring mineral. Furthermore, our state-of-the-art production process is scalable and utilises only environmentally friendly and abundant chemicals. Therefore, it is environmentally sustainable and economically profitable.
How do you control the edges?
In simple words, our process creates holes in the material and thereafter these holes are exposed to a liquid solution. Depending on how long the pre-made holes are exposed to this liquid solution, the closer to our unique hexagonal shape the holes will approach.
Who are your target customers?
SMENA's target customers are gas sensor companies that offer or are looking to expand their portfolio into MOS-gas sensors, able to detect low concentrations of H2, NH3 and NO2. For our catalysis track, we are currently focusing our efforts on producers of PEM-electrolysers and Membrane Electrode Assemblies (MEAs).
References
Munkhbat, Battulga, et al. "Transition metal dichalcogenide metamaterials with atomic precision." Nature communications 11.1 (2020): 1-8. Transition metal dichalcogenide metamaterials with atomic precision | Nature Communications
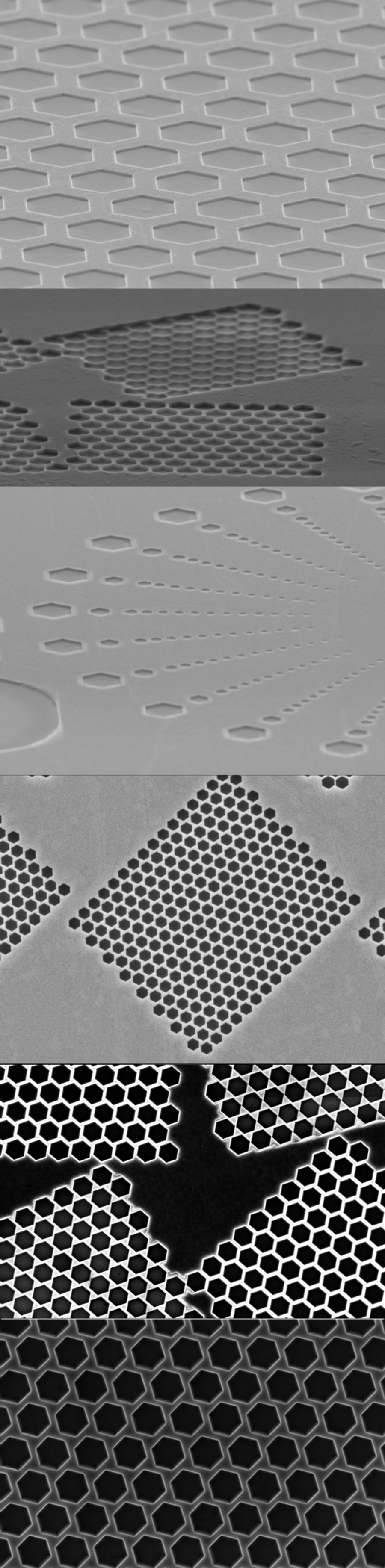
Electron microscopy images exemplify SMENA Catalysis AB products with signature hexagonal holes in MoS2-based material, Molybdenyx. The edges of these holes are atomically sharp "zigzag edges".




